Adhering to the innovativeculture of seeking novelty and truth, and sticking to technologicalempowerment, Tianjin Pharmaceutical Holdings Co., Ltd. is committed to becominga leader in the health industry. At present, it has initially built ascientific and technological innovation system with 2 academician workstations,2 nationwide enterprise technology centers, 6 municipal-level enterprise keylaboratories and 8 municipal-level enterprise technology centers as the mainbody.
It has established fourR&D centers covering the fields of innovative drugs, chemical genericdrugs, traditional Chinese medicine onehealth and medical devices, with nearly1,000 R&D personnel, including 6 experts enjoying special allowances fromthe State Council.
It has undertaken more than100 national and provincial-level science and technology projects, won morethan 10 first and second prizes for national scientific and technologicalprogress, and won provincial-level science and technology awards. It hasestablished scientific research cooperation relations with Tsinghua University,Tianjin Institute of Industrial Biotechnology of China Academy of Sciences,Institute of Biomedical Engineering of China Academy of Medical Sciences,Tianjin University of Traditional Chinese Medicine, Modern Traditional ChineseMedicine Haihe Laboratory and other well-known R&D institutions.
- 2
Academician workstation/Nr
- 2
National enterprise technology center/Nr
- 6
Municipal -level enterprise key laboratory/Nr
- 8
Municipal enterprise technology center/Nr
- Innovative Drug R&D Center
- Pharmaceutical Chemicals R&D Center
- Traditional Chinese Medicine onehealth R&D Center
- Medical Device R&D Center

-
Innovative Drug R&D Center
The R&D Center for Innovative Drugs focuses on eight therapeutic fields, such as self-immunity system, respiration, skin and ophthalmology. takes "endogenous+exogenous" as its main business development model around the direction of frontier development of bio-innovative drugs, and deeply explores and selects high-quality projects of innovative drugs from a global perspective. It has established a pharmacological technology platform and a drug analysis and research platform, and established a new drug screening and design platform based on artificial intelligence AI jointly with Nankai University. It has started seven innovative drug researches, one of which has been declared for production.

-
Pharmaceutical Chemicals R&D Center
The Pharmaceutical Chemicals R&D Center has set up two domestic sub-centers and one overseas sub-center, which complement each other and have the R&D capabilities in line with international standards. The research of pharmaceutical chemicals involves 16 categories in the field of treatment, covering systemic anti-infection, cardiovascular system, endocrine metabolism and so on.Five technical platforms have been successfully built up-covering raw materials, solid preparations, aseptic preparations, external preparations and inhalation preparations. In the short term, we will focus on new products of hormones and amino acids, consistency evaluation of drugs in short supply, innovation of drugs for external use and quality improvement. In the mid-long term, we will focus on the launching of respiratory administration and high-end products for ophthalmology. We have obtained 381 drug approvals and 70 API approvals.

-
Traditional Chinese Medicine onehealth R&D Center
The Traditional Chinese Medicine onehealth R&D Center adopts the mode of new drug research and secondary development in parallel, and complementary and coordinated development of drugs and health care products.The TCM research involves 14 treatment fields, covering cardiovascular and cerebrovascular diseases, respiration, spleen and stomach, etc. At present, it owns 599 approvals for drugs, of which 122 are for exclusively produced drugs and 254 are for drugs covered by medical insurance.拥有多项资质荣It has won a number of qualifications and honors, including national enterprise technology center once, academician workstation once, provincial enterprise key laboratory once, municipal enterprise technology center for five times, enterprise postdoctoral workstation, and nationally recognized (CNAS) testing laboratory etc..
-
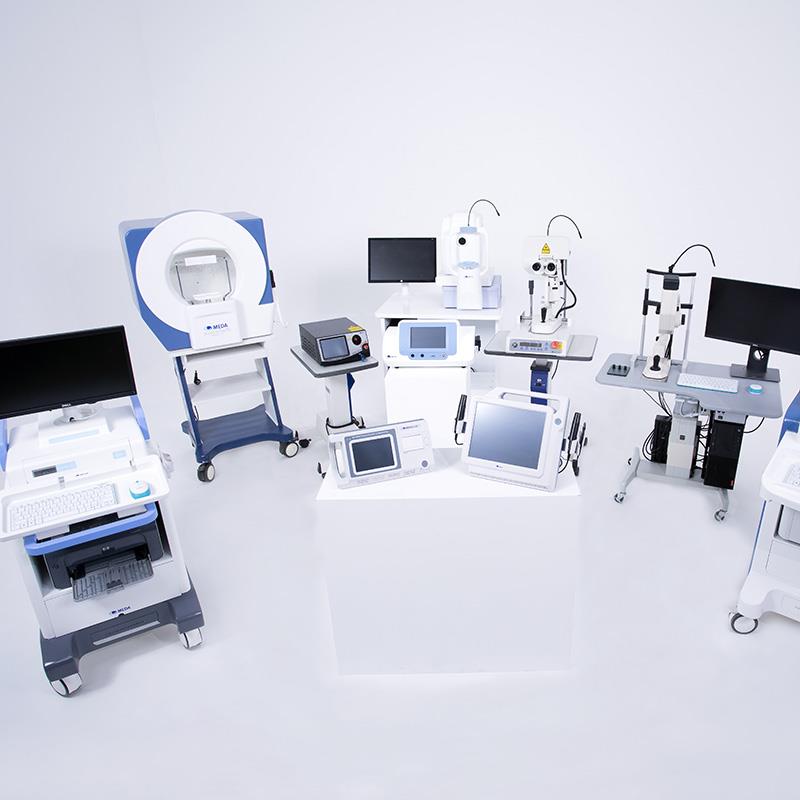
Medical Device R&D Center
The Medical Device R&D Center runs in a multi-field collaborative development model of medical treatment equipment, in-vitro diagnostic medical devices, in-vitro diagnostic reagents and disinfection products, involving multiple treatment fields such as ophthalmology, skin, urology, pediatrics, cardiovascular and cerebrovascular diseases and infectious diseases. Several medical devices have full independent intellectual property rights, replacing the imported devices.
-

-
Obtained domestic unanimous praise
Steroid hormone innovation platform
-

-
Led by enterprise key laboratory
Innovative platform for inhaled drugs
-

-
Established a scientific and reasonable
TCM quality evaluation system and evaluation standardsSecondary development platform for proprietary Chinese medicine


 Tianjin public network security No. 12010202000359
Tianjin public network security No. 12010202000359
